Mainstay Products
Renal diseases and Hemodialysis
Torii mainly markets products related to CKD (chronic kidney disease) in the area of renal diseases and Hemodialysis.
ENAROY Tablets (a renal anemia drug) that Torii launched in December 2020 is an oral drug to improve renal anemia of CKD (chronic kidney disease) patients(dialysis/ peritoneum/conservative phase of kidney disease). ENAROY Tablets promotes erythropoiesis through a mechanism different from existing treatments. ENAROY Tablets stabilizes Hypoxia Inducible Factor(HIF) that attracted much attention at the Nobel Prize in Medicine and Physiology in 2019.
Riona Tablets that Torii launched in May 2014 is a drug with two indications: “improvement of hyperphosphatemia in CKD patients” and “iron deficiency anemia (IDA).” As a therapeutic drug for hyperphosphatemia, Riona Tablets is Japan’s first phosphate binder with iron as its main component. The drug has been confirmed to decrease phosphorus concentrations in the blood of CKD patients by excreting food-derived phosphorous. Riona Tablets obtained additional approval for the treatment of IDA in March 2021. It has been confirmed to improve anemia and replenish iron by elevating the levels of hemoglobin and serum ferritin in IDA patients.
REMITCH CAPSULES (an oral antipruritic drug) that Torii launched in March 2009 is an oral selective opioid kappa receptor agonist for pruritus that is resistant to existing therapies (existing antipruritic drugs are not sufficiently effective) of patients on dialysis, and is highly evaluated as a therapeutic agent for pruritus for patients on dialysis.
REMITCH OD Tablets that Torii launched in June 2017, can be taken with or without water and therefore is convenient for patients whose swallowing capabilities have deteriorated or those who have restrictions on water intake, and helps improve medication adherence.
-
- ENAROY Tablets
- Therapeutic agent for Anemia Associated with CKD
-
- Riona Tablets
-
Therapeutic agent for hyperphosphatemia
/Therapeutic drug for iron deficiency anemia (IDA)
-
- REMITCH
- Oral therapeutic agent for pruritus
Skin diseases
In the area of skin diseases, Torii markets products related to the treatment of skin diseases such atopic dermatitis and tinea pedis.
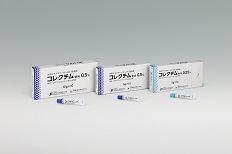
CORECTIM® Ointment 0.5% that Torii launched in June 2020 and CORECTIM® Ointment 0.25% that Torii launched in June 2021 are the world's first topical JAK inhibitor that improves atopic dermatitis by inhibiting the action of JAKs, which play a key role in immune activation signaling in cells, by suppressing the overactivation of immune responses.
In March 2021, CORECTIM® Ointment 0.25% was approved for manufacturing and marketing in Japan for an indication of pediatric atopic dermatitis. In addition, CORECTIM® Ointment 0.5% was approved for additional pediatric dosage and administration.
In January 2023, clinical trial results for patients with atopic dermatitis aged 6 months to 2 years were added to the drug package insert.
ANTEBATE has three dosage forms: ointment, cream, and lotion. It improves the symptoms of skin diseases such as atopic dermatitis and contact dermatitis by reducing inflammation. The availability of three dosage forms allows users to use each one depending on the disease, site, and symptoms. In addition, Torii has made efforts to improve the quality of life (QOL) of patients by improving the quality of the base and ease of use, and ANTEBATE is highly evaluated.
-
- CORECTIM
- Topical Janus kinase (JAK) inhibitor
-
- ANTEBATE
- Topical corticosteroid
Allergens
In the area of allergy, Torii has manufactured and marketed the diagnostic and therapeutic products for allergic diseases for about half a century. Torii currently produces 68 products in both diagnosis and treatment for 37 allergens as April 2023.
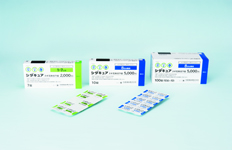
CEDARCURE ® Japanese Cedar Pollen Sublingual Tablets launched by Torii in June 2018 is the sublingual immunotherapy drug for Japanese Cedar Pollinosis and regulatory approved sublingual tablet available for adult and pediatric patients.
MITICURE ® House Dust Mite Sublingual Tablets launched by Torii in December 2015 is an allergen immunotherapy tablet for house dust mite allergy, for the indication of allergic rhinitis. In addition, this drug was approved an additional dosage and administration for pediatric indication in February 2018.
-
- CEDARCURE ® Japanese Cedar Pollen Sublingual Tablets
- Allergen immunotherapy tablet
-
- MITICURE® House Dust Mite Sublingual Tablets
- Allergen immunotherapy tablet